Latest news
 Syracuse Ny Local News - Dec 18th, 2024
Syracuse Ny Local News - Dec 18th, 2024
Syracuse women’s basketball vs. Binghamton won’t be on TV: Here’s where you can still stream it live
The clash of Upstate New York schools will be exclusive to just one streaming service.
No. 1 pick Sarah Fillier already ‘leading charge’ for Sirens heading into home opener
Sarah Fillier was almost in disbelief when Billie Jean King announced that the New York Sirens would be taking Fillier with the No. 1 pick at the June draft.

 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
Draganfly Completes First Proof-of-Concept Flights in Drone Delivery Research Project for Mass General Brigham
Draganfly’s medical drone delivery infrastructure offers new model for future improvement in service time for Home Hospital care

 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
Streamline Health® Expands Presence In Texas with 400-Bed Health System for RevID
Oracle Health partnership enables 400-bed Texas-based health system to leverage RevID’s automated charge reconciliation to improve financial performance Oracle Health partnership enables 400-bed Texas-based health system to leverage RevID’s automated charge reconciliation to improve financial performance

 Syracuse Ny Local News - Dec 18th, 2024
Syracuse Ny Local News - Dec 18th, 2024
Section III girls ice hockey stats leaders (through Dec. 17)
Stats reported to syracuse.com by Section III coaches.
 Syracuse Ny Local News - Dec 18th, 2024
Syracuse Ny Local News - Dec 18th, 2024
Asking Eric: Fostering appreciation in long-term relationships through communication
Start your mornings with Asking Eric.

 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
Asia Pacific Aesthetic Medicine Market Set to Surpass Valuation of US$ 65.42 Billion By 2033 | Astute Analytica
Asia Pacific’s aesthetic medicine market is a goldmine of opportunity, driven by evolving consumer preferences and groundbreaking advancements. This vibrant sector is poised to secure impressive returns and establish a strong foothold in a rapidly growing industry. Asia Pacific’s aesthetic medicine market is a goldmine of opportunity, driven by evolving consumer preferences and groundbreaking advancements. This vibrant sector is poised to secure impressive returns and establish a strong foothold in a rapidly growing industry.

 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
The Future of Aircraft Health Monitoring: Market Forecast and Growth Opportunities to 2028
Delray Beach, FL, Dec. 18, 2024 (GLOBE NEWSWIRE) -- The Aircraft Health Monitoring Market size is projected to grow from USD 6.3 Billion in 2023 to USD 8.6 Billion by 2028, at a CAGR of 6.5% from 2023 to 2028.

 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
Corvus Pharmaceuticals Announces Interim Data from Placebo-Controlled Phase 1 Clinical Trial of Soquelitinib for Atopic Dermatitis
Data from lowest dose level cohorts demonstrate a favorable safety and efficacy profile
 Mlive - Dec 18th, 2024
Mlive - Dec 18th, 2024
A chain restaurant closes, Western wear store opens and more local business news
From a temporary closure at UNO Pizzeria & Grill to a new western wear store opening in time for your last-minute holiday shopping, here’s some recent local business news you might have missed.

 Mlive - Dec 18th, 2024
Mlive - Dec 18th, 2024
73k acres of Upper Peninsula wilderness preserved with $4.2M trust fund award
Major conservation project in the Upper Peninsula gets largest grant in latest trust fund recommendations.

 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
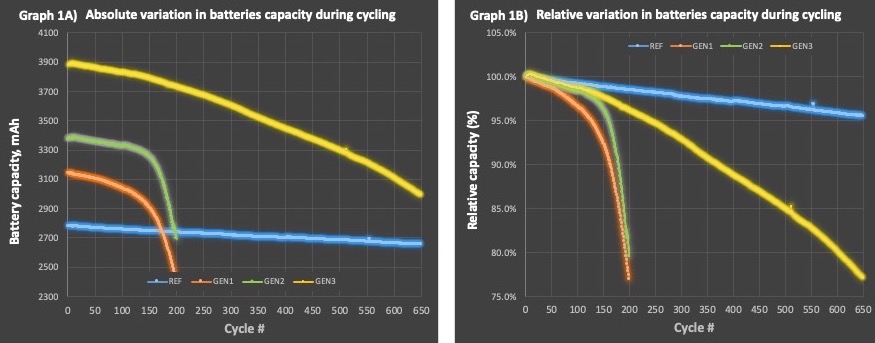
Novacium’s Silicon-Anode Batteries Show Superior Cumulative Energy Return Over 650 Cycles—Compared to High-Grade Artificial Graphite
MONTREAL, Dec. 18, 2024 (GLOBE NEWSWIRE) -- HPQ Silicon Inc. (“HPQ” or the “Company”) (TSX-V: HPQ, OTCQB: HPQFF, FRA: O08), a technology company specializing in green engineering of silica and silicon-based materials, is pleased to update shareholders on the latest battery milestones achieved by its France-based affiliate, NOVACIUM SAS (Novacium).
 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
LeddarTech présente ses résultats pour l’exercice 2024
QUÉBEC, Canada, 18 déc. 2024 (GLOBE NEWSWIRE) -- LeddarTech® Holdings Inc. (« LeddarTech ») (NASDAQ : LDTC), une société de logiciels automobiles qui fournit des technologies logicielles de fusion bas niveau de capteurs et de perception reposant sur l’IA, innovatrices et brevetées pour systèmes avancés d’aide à la conduite (systèmes ADAS), de conduite autonome (systèmes AD) et de stationnement, est heureuse d’annoncer ses résultats financiers pour l’exercice 2024, qui s’est terminé le 30 septembre 2024.

 Mlive - Dec 18th, 2024
Mlive - Dec 18th, 2024
After tearing down blighted apartments, Genesee County OKs sale to McLaren Flint hospital
Deb Cherry served 14 years as Genesee County treasurer and wanted to check one more thing off her to-do list before leaving her post at the end of this year.
 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
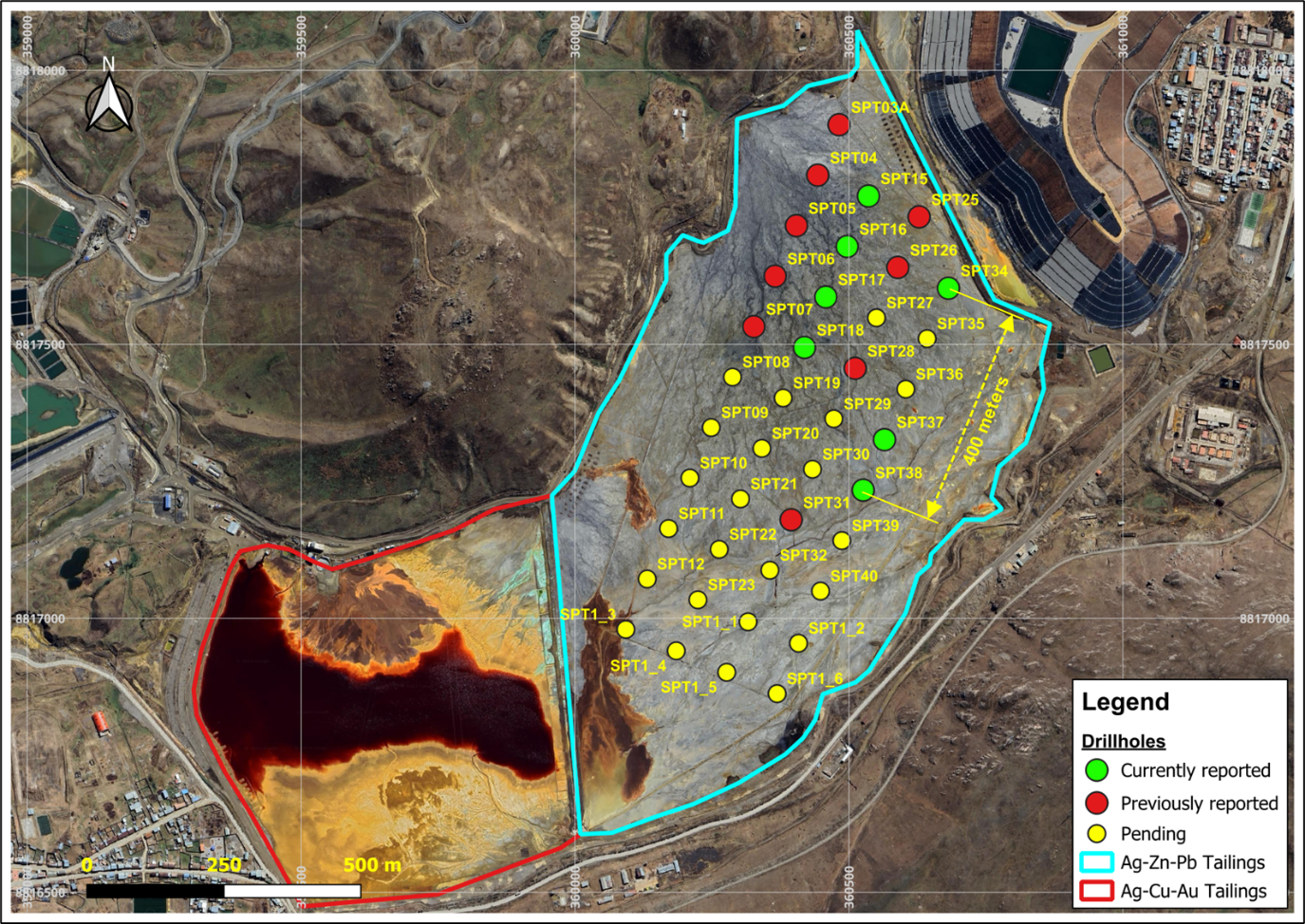
Cerro de Pasco Resources Reports Additional Results from the Quiulacocha Silver-Zinc-Lead Zone
Cerro de Pasco Resources Inc. reports assay results for an additional seven drill holes from the Quiulacocha Tailings Project in Central Peru.
 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
Perma-Fix Announces Pricing of $22 Million Public Offering of Common Stock
ATLANTA, Dec. 18, 2024 (GLOBE NEWSWIRE) -- Perma-Fix Environmental Services, Inc. (Nasdaq: PESI) (“Perma-Fix” or the “Company”), today announced the pricing of its previously announced underwritten public offering of 2,200,000 shares of its common stock at a price to the public of $10.00 per share. Perma-Fix expects the gross proceeds from the offering to be approximately $22 million before deducting the underwriting discount and other estimated offering expenses. In connection with the offering, Perma-Fix has granted the underwriter a 30-day option to purchase up to 330,000 additional shares of its common stock at the public offering price, less the underwriting discount. The offering is expected to close on or about December 19, 2024, subject to the satisfaction of customary closing conditions.

 Benzinga - Dec 18th, 2024
Benzinga - Dec 18th, 2024
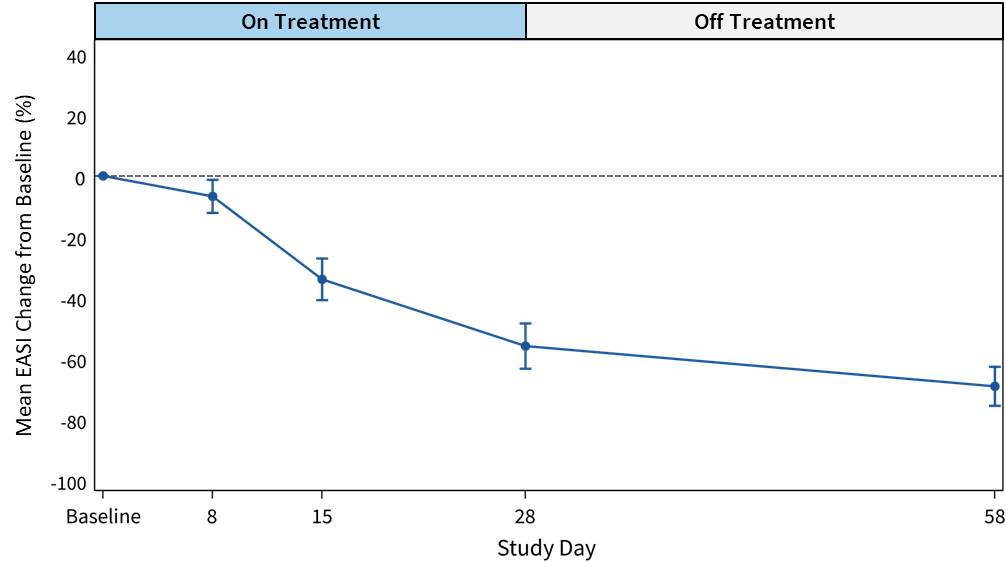
Corvus Pharmaceuticals Announces Interim Data from Placebo-Controlled Phase 1 Clinical Trial of Soquelitinib for Atopic Dermatitis
Data from lowest dose level cohorts demonstrate a favorable safety and efficacy profileData includes complete results from cohort 1 and initial results from cohort 2Early exercise of common stock warrants from stockholder generates cash proceeds of approximately $12.7 millionCompany to host conference call and webcast today at 8:00 a.m. ET / 5:00 a.m. PTBURLINGAME, Calif., Dec. 18, 2024 (GLOBE NEWSWIRE) -- Corvus Pharmaceuticals, Inc. (NASDAQ:CRVS), a clinical-stage biopharmaceutical company, today announced interim data from the randomized, double-blind, placebo-controlled Phase 1 clinical trial evaluating soquelitinib in patients with moderate to severe atopic dermatitis. The data demonstrated a favorable safety profile and efficacy profile, supporting the ongoing development of soquelitinib for atopic dermatitis and the potential of ITK inhibition as a novel mechanism of action for other immune diseases."We are pleased with the early results of our soquelitinib Phase 1 atopic dermatitis clinical trial, which show an attractive potential product profile at the lowest dose we are studying," said Richard A. Miller, M.D., co-founder, president and chief executive officer of Corvus. "The data show consistent signs of efficacy, combined with a novel mechanism of action, a convenient oral route of administration and a favorable safety profile. This is also supported by an analysis of serum cytokine levels, which show a possible relationship between clinical response and reductions in IL-5, IL-17, IL-31, IL-33 and TSLP, along with a trend for TARC. We believe the data highlights soquelitinib's potential as a new treatment option for atopic dermatitis and the broader opportunity for ITK inhibition for other immune related diseases. In addition to blocking the production of multiple inflammatory cytokines, soquelitinib may have persistent direct effects on immune cell function that act to regulate aberrant immune responses. We look forward to completing the Phase 1 trial and initiating other trials with soquelitinib for immune diseases."Soquelitinib Atopic Dermatitis Phase 1 Clinical Trial DesignThe randomized, double-blind, placebo-controlled Phase 1 clinical trial is planned to enroll 64 patients with moderate to severe atopic dermatitis that previously failed one prior topical or systemic therapy. Patients are enrolled into one of four dosing cohorts in a 3:1 ratio (12 active and 4 placebo) to receive either soquelitinib or placebo. The cohorts are sequentially enrolled and will examine 100 mg oral twice per day, 200 mg oral once per day, 200 mg oral twice per day and 400 mg oral once per day. Patients are treated for 28 days and are then followed for an additional 30 days with no therapy.These doses were selected based on the Company's prior experience evaluating soquelitinib in T cell lymphoma patients. The doses in the atopic dermatitis trial bracket the 200 mg oral twice a day dosing regimen, which is the level that has been shown to provide complete ITK occupancy and that is being evaluated in the Company's ongoing registrational Phase 3 clinical trial of soquelitinib in peripheral T cell lymphoma.The primary endpoints include safety and tolerability, and efficacy, measured by improvement in Eczema Area and Severity Index (EASI) score, Investigator Global Assessment (IGA), reduction in itch and various cytokine biomarkers. EASI scores are also evaluated by the percent of patients that achieve a specified percent reduction in EASI score – EASI 50 for patients that achieved a 50% reduction; EASI 75 for a 75% reduction; and EASI 90 for a 90% reduction. Corvus and a data monitoring committee will be able to monitor the data from the trial as the trial progresses.Soquelitinib Interim Data from the Atopic Dermatitis Phase 1 Clinical TrialThe Company is reporting complete results from Cohort 1 of the trial, which includes 16 patients (12 that received soquelitinib 100 mg oral twice per day and four that received placebo) with follow up at 28 days and at 58 days. At 58 days, two patients in the soquelitinib group were not available for follow up. The soquelitinib and placebo patients were well matched; see Table 1 below for patient characteristics. Table 1: Cohort 1 Patient Characteristics SoquelitinibPlacebo (N=12)(N=4)Age, mean (range), yrs46.3 (30–66)50.5 (32–62)Gender, male n (%)7 (58.3)4 (100)Race/ethnicity, n (%) Asian2 (16.7)0 (0) Black or African American6 (50)4 (100) White3 (25)0 (0) Hispanic or Latino1 (8.3)0 (0)Baseline EASI, mean (range)20.4 (15.0–46.6)18.5 (14.9–24.8)Baseline IGA, mean (range)3.0 (2–4)3.3 (3–4)Prior AD therapies, n (%) Topical Corticosteroids11 (91.7)4 (100) Systemic therapies3 (25)Full story available on Benzinga.com

 Benzinga - Dec 18th, 2024
Benzinga - Dec 18th, 2024
Perma-Fix Announces Pricing of $22 Million Public Offering of Common Stock
ATLANTA, Dec. 18, 2024 (GLOBE NEWSWIRE) -- Perma-Fix Environmental Services, Inc. (NASDAQ:PESI) ("Perma-Fix" or the "Company"), today announced the pricing of its previously announced underwritten public offering of 2,200,000 shares of its common stock at a price to the public of $10.00 per share. Perma-Fix expects the gross proceeds from the offering to be approximately $22 million before deducting the underwriting discount and other estimated offering expenses. In connection with the offering, Perma-Fix has granted the underwriter a 30-day option to purchase up to 330,000 additional shares of its common stock at the public offering price, less the underwriting discount. The offering is expected to close on or about December 19, 2024, subject to the satisfaction of customary closing conditions.Perma-Fix intends to use the net proceeds from the offering to fund (i) continued R&D and business development relating to the Company's patent-pending Perma-FAS process for the destruction of PFAS, as well as the cost of installing at least one second-generation Perma-FAS commercial treatment unit; (ii) ongoing facility cap-ex and maintenance costs; as well as (iii) general corporate and working capital purposes.Craig-Hallum is acting as sole managing underwriter for the offering. Wellington Shields is acting as financial advisor to the Company for the offering.The shares described above are being offered by Perma-Fix pursuant to a shelf registration statement on Form S-3 (File No. 333-283555), including a base prospectus, that was filed with the Securities and Exchange Commission (SEC) and declared effective on December 12, 2024. The offering is being made only by means of a prospectus supplement, ...Full story available on Benzinga.com

 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
Amesite Announces NurseMagicTM Enterprise Contract Wins in High Growth Companies with Hundreds of Franchise Owners
Company Generating Recurring Revenue in $330 Billion Revenue Home Health and Home Care Industries Company Generating Recurring Revenue in $330 Billion Revenue Home Health and Home Care Industries

 Globe Newswire - Dec 18th, 2024
Globe Newswire - Dec 18th, 2024
Mesoblast to be Added to Nasdaq Biotechnology Index
NEW YORK, Dec. 18, 2024 (GLOBE NEWSWIRE) -- Mesoblast Limited (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today announced its upcoming addition to the Nasdaq Biotechnology Index (Nasdaq: NBI) as part of the annual reconstitution of the 2024 Nasdaq index. Mesoblast’s inclusion in the NBI will be effective after the U.S. market opens on Monday, December 23, 2024.
